BACKGROUND
Addressing unmet needs
in CVD
Inflammation is a primary driver in a variety of disease states and adversely affects human health in a myriad of ways.
At ResoTher Pharma, we focus on endogenous immune regulatory pathways in the propagation and resolution of inflammation.
We work to validate a class of novel therapeutic peptides in critical-care cardiovascular indications, where tissue injury is linked to exacerbated inflammation and poor prognosis.
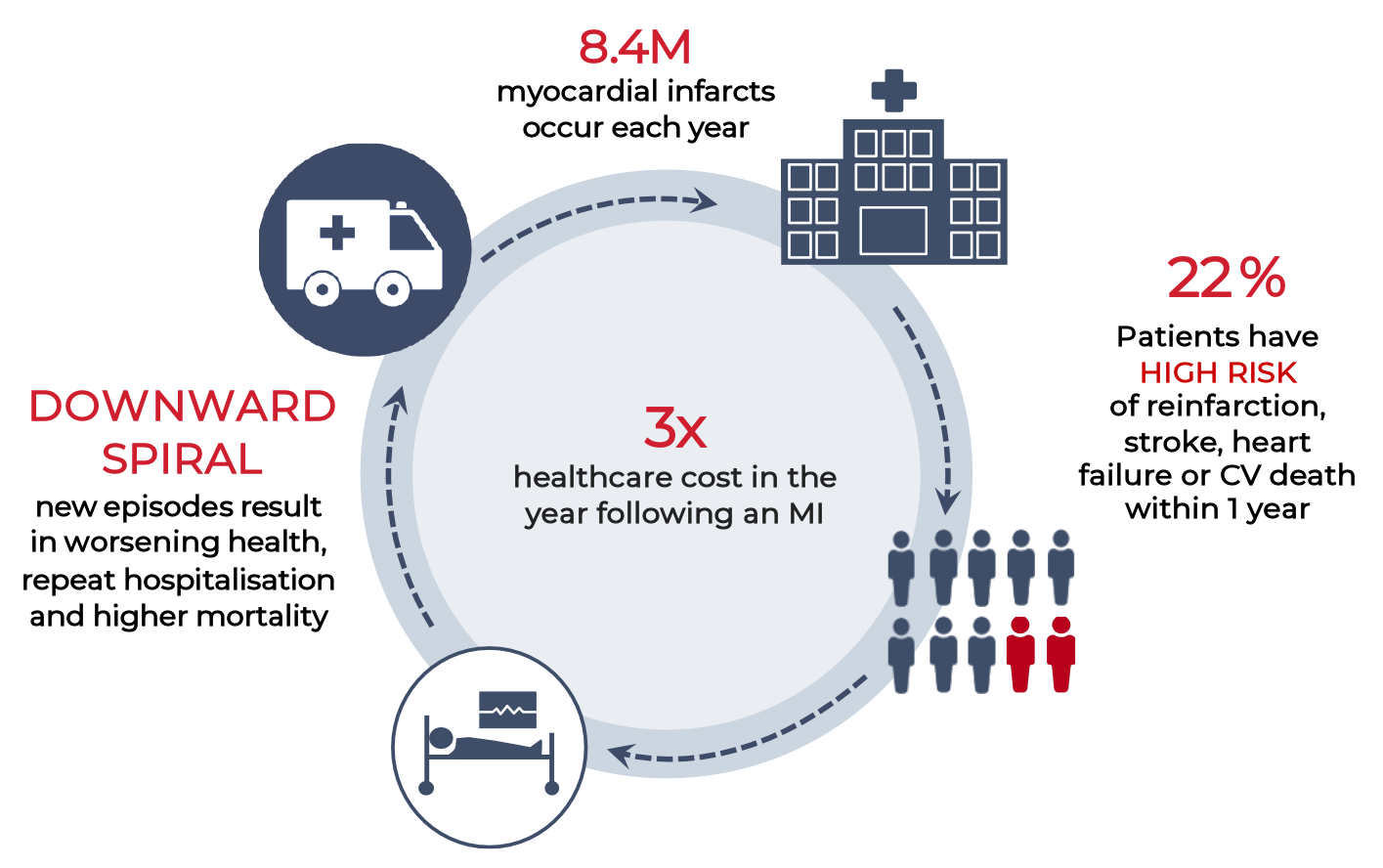
MYOCARDINAL INFARCTION
Current standard of care leaves a treatment gap
SCIENCE
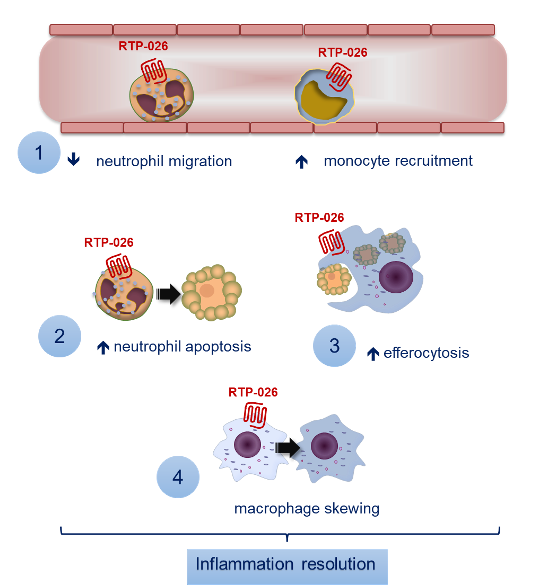
Novel approach to activate repair circuits
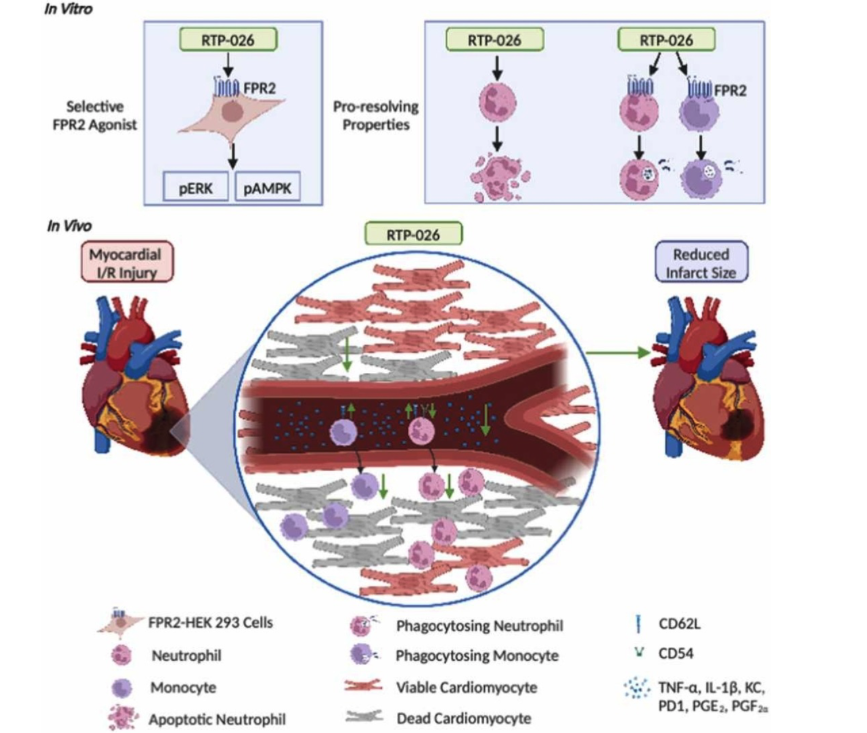
Our drug candidate, RTP-026, is derived from the endogenous human protein Annexin A1. This protein has documented effects on resolution of inflammation and provides tissue protection in a variety of inflammation-driven disease models.
RTP-026 mimics the action of Annexin A1 and initiates resolution and tissue repair through its action on a G-protein coupled receptor expressed on the surface of leukocytes.
Mechanism
of action
RTP-026 activates tissue reparative pathways by targeting innate immune cells. Our preclinical data shows that RTP-026 works by:
- Regulating neutrophil and monocyte recruitment to injured tissue
- Promoting neutrophil apoptosis and thereby limiting destruction of surrounding tissue
- Removing apoptotic cells through efferocytosis
- Promoting macrophage switch towards a pro-resolving phenotype.
Together, these orchestrated cellular events contribute to resolution of inflammation and activation of cellular event that support tissue healing.
Publications
Chen, J. et al. (2023). The Annexin-A1 mimetic RTP-026 promotes acute cardioprotection through modulation of immune cell activation. Pharmacol Res. 198:107005.
PIPELINE
Potent immune modulators with excellent safety
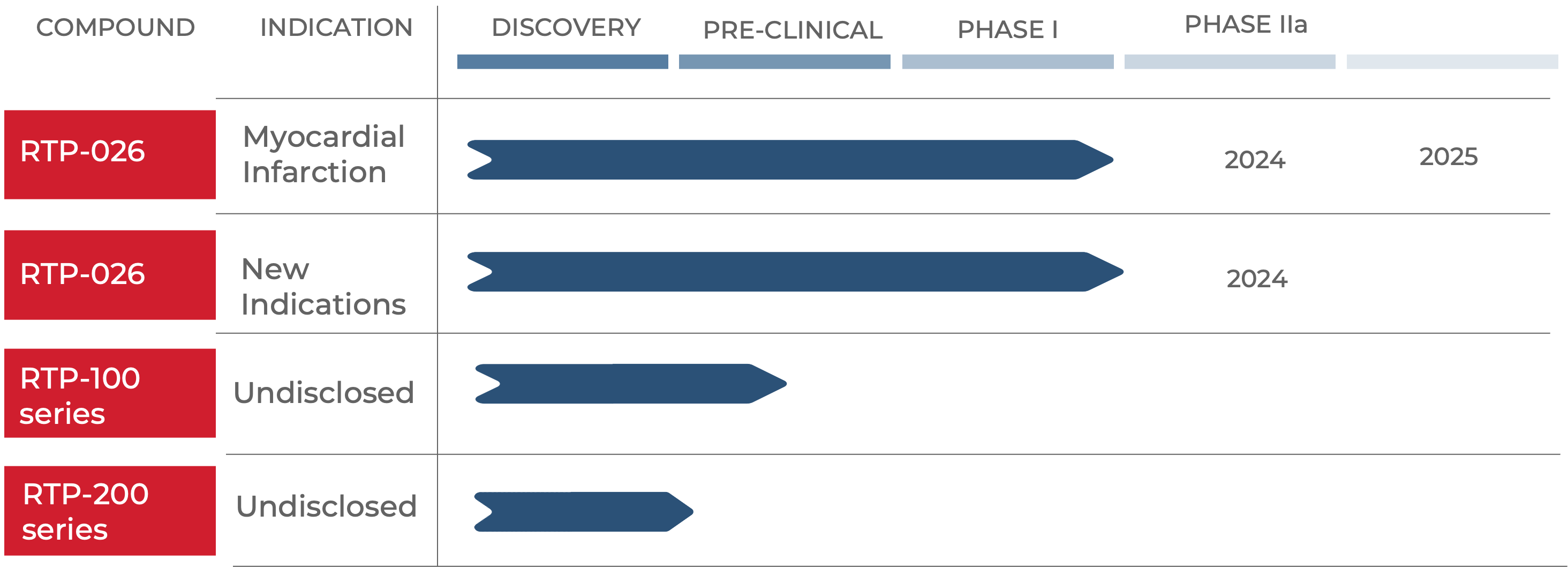
Our focus is on advancing our novel peptide drugs into clinical development for acute, critical care cardiovascular indications.
Our lead drug candidate, RTP-026, has shown excellent efficacy in a variety of inflammation-driven disease models and was demonstrated to have an excellent safety profile in a first in man Phase I, double-blind, placebo-controlled study in healthy male volunteers and in one group of post-menopausal women.
Our first patient study will commence in 2024 and will test the effects of RTP-026 in patients with myocardial infarction.
Collaboration
Current treatment for cardiovascular disease is inadequate, leaving a gap for new, targeted therapies to tackle the inflammatory component of disease. ResoTher Pharma aims to fill this gap with our novel Resolution Therapies.
We take an active partnering approach and welcome inquiries for collaboration around our assets.”